ECMO centrum
OSIRIS ECPR trial
OSIRIS ECPR trial: Out-of-hospital refractory cardiac arrest treated with extracorporeal cardiopulmonary resuscitation, a multicenter, randomized, controlled trial
What is OSIRIS ECPR trial
OSIRIS ECPR trial is a multicenter, randomized controlled trial to assess effectiveness of extracorporeal cardiopulmonary resuscitation (ECPR) based system versus conventional cardiopulmonary resuscitation (CCPR) in refractory out-of-hospital cardiac arrest, demonstrate its economic effectiveness and drive acceptance by patients, professionals and health systems.
The trial aims not only to determine whether ECPR improves outcomes, but also to assess whether this advanced therapy can be safely, efficiently, and equitably implemented across different European healthcare systems, thereby supporting informed decision-making by patients, clinicians, health authorities, and policymakers.
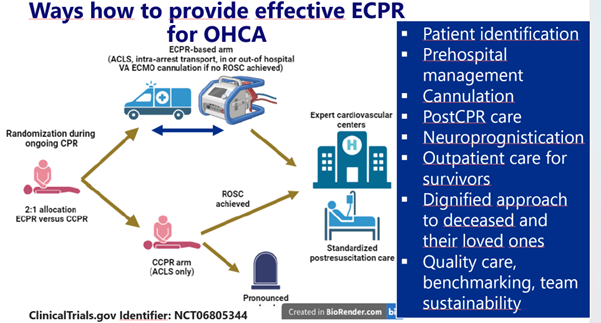
We hypothesize that ECPR, delivered within a standardized and protocolized system of care, improves survival and neurological recovery in patients who do not respond to conventional CPR.
Our core research question is: Does an ECPR-based treatment strategy, implemented within a protocolized system, improve 90-day survival compared with CCPR alone in adults with refractory OHCA due to an initial shockable rhythm?
The trial has been registered at ClinicalTrials.gov (NCT06805344), study scheme is depicted in Figure 1.
Cardiac arrest – a major burden for Europe
Out-of-hospital cardiac arrest (OHCA) is a leading cause of death in Europe, affecting over 300,000 people annually1 and is recognized as a high-priority public health topic. OHCA is a leading cause of economic productivity loss and must become a focus of public health policy. The mean annual and lifetime productivity losses per OHCA in 2018 were ~ €45,000 and €595,000 respectively. The total annual and lifetime economic productivity loss due to OHCA in the U.S. was €10.5 and $139.8B in 2018, respectively.1 Sudden cardiac death is responsible for half of all heart disease deaths. Despite considerable efforts to enhance outcomes, survival upon hospital discharge remains distressingly low, with an average of 8%.2 Survival with a good neurological and functional recovery are even less frequent. Therefore, a new multinational project to possibly improve OHCA outcome and be implemented around Europe is urgently needed, see Figure 2.
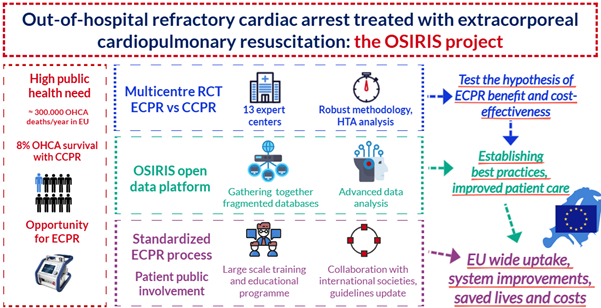
Study background
Conventional cardiopulmonary resuscitation (CCPR) is well-established across most European countries; however, it has reached its limitations in improving survival. Extracorporeal cardiopulmonary resuscitation (ECPR) has emerged as a promising approach to improve outcomes.3-5 ECPR utilizes veno-arterial extracorporeal membrane oxygenation (V-A ECMO) during CPR, which restores perfusion to vital end-organs and stabilizes the patient for interventions that address the underlying cause of arrest. To adequately assess benefits of ECPR for out-of-hospital. Although promising, ECPR is not yet widely adopted, and its benefits and risks are not fully understood. The OSIRIS trial is the first large, international, randomized study comparing ECPR with standard CPR in patients with refractory cardiac arrest—those whose hearts do not restart after the initial CPR attempts.
Participating centres
The study includes 13 high-volume cardiac arrest centres with established ECPR protocols.
| ERA4Health No | Research Partners | Role | Short Name | Country |
| 1 | GENERAL UNIVERSITY HOSPITAL | Coordinator | GUH | CZ |
| 2 | MEDICAL UNIVERSITY OF VIENNA | Partner | MUV | Austria |
| 3 | VALL D’HEBRON RESEARCH INSTITUTE | Partner | VHIR | Spain |
| 4 | WROCLAW MEDICAL UNIVERSITY | Partner | WMU | Poland |
| 5 | UNIVERSITY HOSPITAL DÜSSELDORF | Partner | UDUS | DE |
| UNIVERSITY HOSPITAL FREIBURG | Recruiting sites | DE | ||
| CHARITE HOSPITAL BERLIN | Recruiting sites | DE | ||
| HEART CENTER LEIPZIG AND LEIPZIG HEART SCIENCE | Recruiting sites | DE | ||
| ASKLEPIOS KLINIK HAMBURG | Recruiting sites | DE | ||
| 6 | GUYS AND ST THOMAS’S NHS FOUNDATION TRUST, HAREFIELD HOSPITAL | Partner | UK | |
| MASARYK UNIVERSITY | Project management, Data management | MUNI | CZ | |
| EEN No | External Existing Network Organisation Name | Role | Short Name | Country |
| 1 | UNIVERZITETNI KLINICNI CENTER LJUBLJANA | Recruiting sites | UMCL | SI |
| 2 | INSEL GRUPPE AG | Recruiting sites | INSEL | CH |
| 3 | UNIVERSITÄTSSPITAL ZÜRICH | Recruiting sites | USZ | CH |
Table 1: Participating centers
History of scientific cooperation
OSIRIS ECPR trial is built on long-term scientific cooperation of European high-volume cardiac ECMO centres, which started in 2020 by development of Covid-ECMO database under the mandate of European Chapter of the Extracorporeal Life Support Organization (EuroElso)6,7. This database provided extremely important information during the early stages of Covid pandemics and allowed spreading of clinical experiences with ECMO use in COVID. Many centers followed and joined the worldwide ELSO registry later, which includes also cardiac arrest cases. Following on success of this cooperation, the high-volume cardiac centres met in February 2024, in Frankfurt, at a fist investigator meeting, during which the study protocol was agreed on. Since then, regular annual investigator meetings have been taking place as a part of annual conference ECPR Prague School. This long-term collaboration ensured a strong alignment over the study protocol and strong commitment to execute the trial.
What does the OSIRIS trial mean for patients and families?
For patients suffering a sudden cardiac arrest outside the hospital, every minute matters. Many patients do not respond to standard resuscitation and currently have very limited chances of survival.
The OSIRIS trial aims to determine whether advanced life-support technologies can offer selected patients a real second chance at life, with meaningful neurological recovery and acceptable quality of life. By rigorously evaluating ECPR across different European healthcare systems, this study seeks to ensure that future patients receive the right treatment, at the right time, in the right setting based on solid scientific evidence rather than local availability alone.
Impact beyond survival
While survival is the primary outcome of the OSIRIS trial, survival alone is not sufficient. For patients, families, and society, the quality of survival matters.
OSIRIS therefore places strong emphasis on neurological outcome, functional independence, and health-related quality of life, assessed using validated instruments up to 90 days and beyond. By doing so, the trial aims to determine not only whether patients survive, but how they live after survival. OSIRIS will generate critical data on return to functional independence and societal participation, long-term neurological and cognitive outcomes, Quality-adjusted life years (QALYs), healthcare resource utilization and cost-effectiveness.
These data are essential for understanding the true value of ECPR in modern healthcare systems. By integrating clinical, neurological, and economic outcomes, OSIRIS will provide decision-makers with the evidence needed to balance clinical benefit, patient-centered outcomes, and sustainability of healthcare systems.
Ultimately, the OSIRIS ECPR trial aims to move the field beyond a narrow focus on short-term survival and toward a holistic, patient-centered evaluation of outcomes that matter most to patients, families, and society.
Why Europe?
Out-of-hospital cardiac arrest represents a shared and pressing health challenge across Europe, yet outcomes vary widely between regions due to differences in emergency medical services, hospital infrastructure, access to advanced technologies, and post-resuscitation care.
The OSIRIS ECPR trial addresses a question that cannot be answered by a single country or healthcare system alone. ECPR is a highly complex, resource-intensive intervention whose effectiveness depends not only on the technology itself, but also on system organization, logistics, training, and equity of access. A multinational European trial is therefore essential to determine whether ECPR can be implemented safely, effectively, and reproducibly across diverse real-world settings.
By bringing together leading high-volume cardiac arrest centres from multiple European countries, OSIRIS will evaluate ECPR within different EMS models and healthcare systems, generate evidence that is generalizable across Europe and support harmonization of clinical practice, guidelines, and reimbursement policies.
The results of OSIRIS will directly inform European clinical guidelines, health technology assessment (HTA), and policy decisions, helping to ensure that innovative life-saving therapies are delivered equitably and based on robust evidence.
Funding of the trial
The study is planned to be funded from several resources:
- Patient care: as ECPR as an approved treatment method, the clinical care of patients enrolled in the study is covered by national insurance mechanisms.
- Study cost: The consortium is seeking public funding from the ERA4HEALTH funding scheme. For that purpose, the scientific network is divided into the consortium eligible for the public funding support (10 centres) and External Existing Network (3 centres) which will be funded from other resources. The General University Hospital / the Sponsor has granted an institutional funding of 300000 EUR to cover some of the research cost of the external network. The letter of commitment is available HERE. All External Existing Network centres have confirmed their commitment via letters of support:
References
- Coute RA, Nathanson BH, Kurz MC, DeMasi S, McNally B, Mader TJ. Annual and lifetime economic productivity loss due to adult out-of-hospital cardiac arrest in the United States: A study for the CARES Surveillance Group. Resuscitation. 2021;167:111-117. doi:10.1016/j.resuscitation.2021.07.034
- Gräsner JT, Herlitz J, Tjelmeland IBM, et al. European Resuscitation Council Guidelines 2021: Epidemiology of cardiac arrest in Europe. Resuscitation. 2021;161:61-79. doi:10.1016/j.resuscitation.2021.02.007
- Belohlavek J, Smalcova J, Rob D, et al. Effect of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment on Functional Neurologic Outcome in Refractory Out-of-Hospital Cardiac Arrest: A Randomized Clinical Trial. JAMA. 2022;327(8):737-747. doi:10.1001/jama.2022.1025
- Yannopoulos D, Bartos J, Raveendran G, et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open-label, randomised controlled trial. Lancet. 2020;396(10265):1807-1816. doi:10.1016/S0140-6736(20)32338-2
- Belohlavek J, Yannopoulos D, Smalcova J, et al. Intraarrest transport, extracorporeal cardiopulmonary resuscitation, and early invasive management in refractory out-of-hospital cardiac arrest: an individual patient data pooled analysis of two randomised trials. EClinicalMedicine. 2023;59:101988. Published 2023 May 5. doi:10.1016/j.eclinm.2023.101988
- Lorusso R, Combes A, Lo Coco V, et al. ECMO for COVID-19 patients in Europe and Israel. Intensive Care Med. 2021;47(3):344-348. doi:10.1007/s00134-020-06272-3
- Lorusso R, De Piero ME, Mariani S, et al. In-hospital and 6-month outcomes in patients with COVID-19 supported with extracorporeal membrane oxygenation (EuroECMO-COVID): a multicentre, prospective observational study. Lancet Respir Med. 2023;11(2):151-162. doi:10.1016/S2213-2600(22)00403-9

